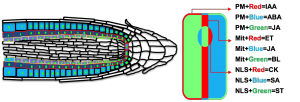
Phytohormones are growth regulators that govern plant development, control interactions with the environment, and orchestrate plant adaptation and survival in ever-changing environments. In the past two decades, a handful of plant biosensors have been developed that enable live or ex vivo imaging of hormone levels and distribution. Synthetic transcriptional reporters, such as EBS for ethylene or DR5 for auxin, have been successfully applied to characterize the effects of various genetic or environmental perturbations on the spatiotemporal activity patterns of individual hormones. The utility of these hormone-specific sensors is, however, limited to detecting one growth regulator at a time. To increase the readout capacity of transcriptional reporters, we are multiplexing several synthetic biosensors in a single construct. Using GoldenBraid molecular cloning technology, we have generated a collection of synthetic hormone-responsive promoters, core promoter elements and terminators, as well as multiple versions of red, green and blue fluorescent proteins and subcellular localization signals. We are in the process of assembling, testing in transient assays and combining various hormone-specific transcriptional units for in planta expression. Our immediate plan is to generate and characterize in Arabidopsis and tomato a single-locus ACE (auxin/ethylene/cytokinin) sensor, with the ultimate goal to multiplex nine transcriptional reporters for nine major growth regulators (ACE plus ABA, gibberellins, brassinosteroids, salicylic acid, jasmonate, and strigolactones), with an individual hormone readout distinguishable by fluorescent protein color and subcellular localization.